CDC Report: Deaths Caused by Alcohol Increased in the US
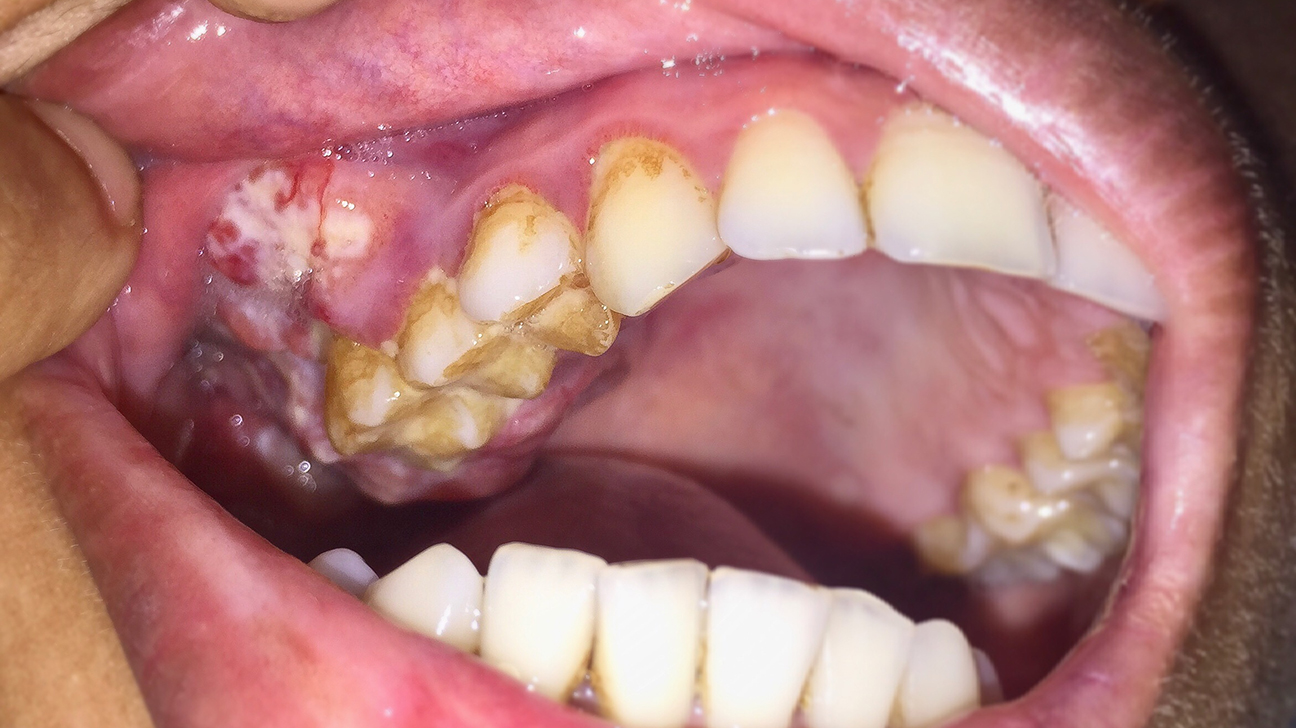
Deaths caused by alcohol consumption increased in the United States during the first year of the COVID-19 pandemic, with the rate of alcohol-related deaths increasing by 26% from 2019 to 2020, the CDC reported. Around 49,000 alcohol-related deaths were recorded in 2020, increasing from around 39,000 in 2019. The US CDC data showed, alcohol caused […]
Continue Reading