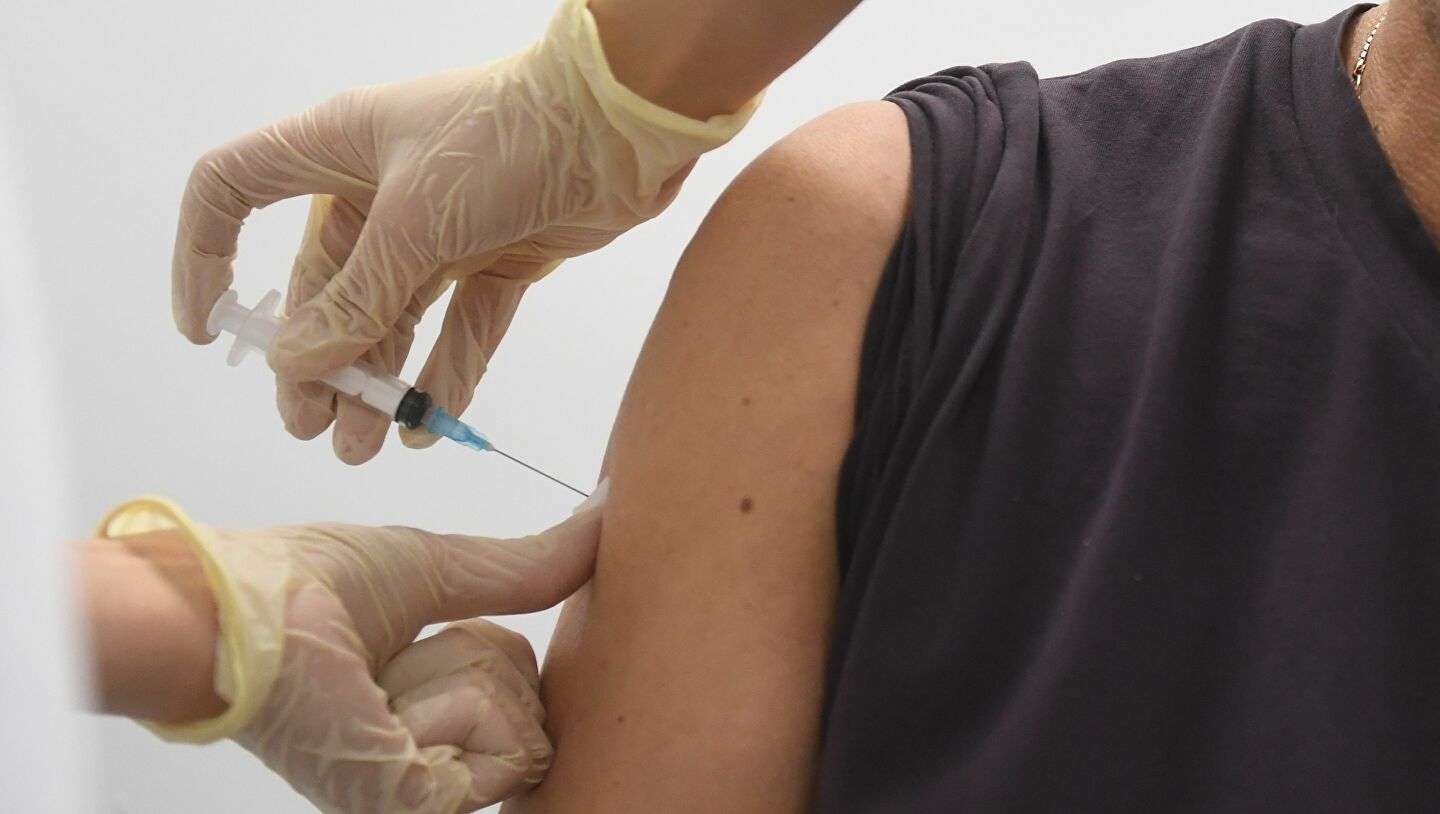
On Thursday, 5th May 2022, the Food and Drug Administration (FDA) of the United States had been limiting the use of the corona virus vaccine of Johnson & Johnson for the adult people because of the risk of a rare blood clotting syndrome.
The corona virus vaccine shot of Johnson & Johnson, which has now received the approval from the United States in the month of February 2021 for the adults in the United States and it can also be administered in the cases where authorized corona virus vaccine have not been accessible and has also been less keen on using the other two shots of ten corona virus vaccine.
The Johnson and Johnson covid-19 vaccine has been one of the tree vaccines in use in the United States and the other two corona virus vaccines have been from Moderna and Pfizer. Johnson & Johnson said that, the company has updated the corona virus vaccine fact sheet of the United States for warning about the risk of thrombosis along with thrombocytopenia syndrome (TTS), which is a rare but a potential life-threatening condition.
The use of the corona virus vaccine shot of Johnson & Johnson has been weak in the countries of high-income, which has been a potentially deadly blood clots, along with the production issues including an accidental mixture of the ingredients by a contract manufacturer and also concerns about the efficacy of the corona virus vaccine of the company.