The US Food and Drug Administration (FDA) of the United States has approved Janssen Pharmaceutical’s IMBRUVICA for the treatment of pediatric patients one year and older suffering with chronic graft-versus-host disease after the failure of more than one therapy.
Janssen claims that, this milestone marks the first pediatric indication for IMBRUVICA, and the launch of a new oral suspension formulation for patients aging one to less than 12 years. Currently, IMBRUVICA is the first therapy approved by the US FDA for younger patients, who had no approved treatment options for the life-threatening disease.
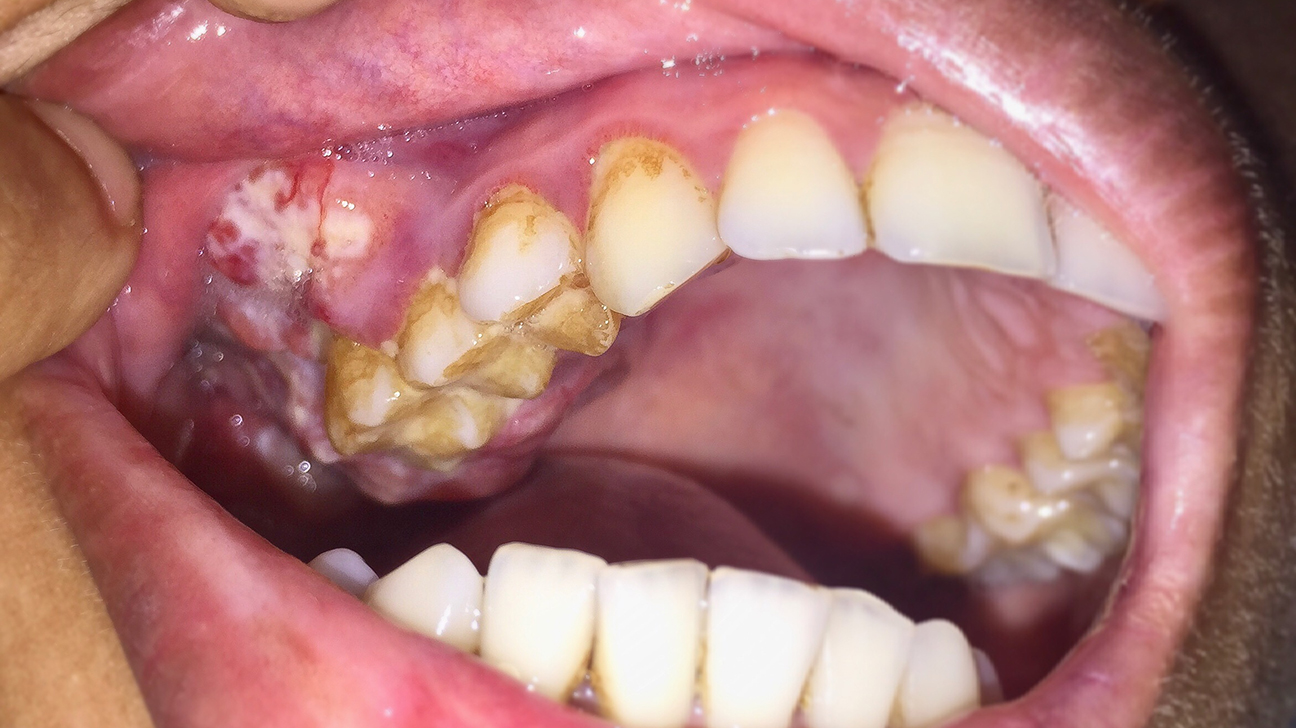
Chronic graft-versus-host disease is a life-threatening disease that can occur after a bone marrow or stem cell transplant when the newly transplanted donor cells have attacked the body of the transplant recipient.
The symptoms of the disease are skin rash, liver inflammation, dry eyes, mouth sores, and development of scar tissue in the joints and skin, and it also cause damage to the lungs.
Dr. Paul A. Carpenter, physician at Seattle Children’s Hospital, said if the children were between one and 12 years old and did not respond to the steroid treatment, they did not have any rigorously studied treatment options.
He said the new IMBRUVICA oral suspension formulation also helps in addressing the challenges might have with the consumption of tablets or capsules.
This approval of IMBRUVICA from the US FDA puts another weapon in the arsenal of children, and has the potential to truly make a difference for the children who are facing this challenging disease.