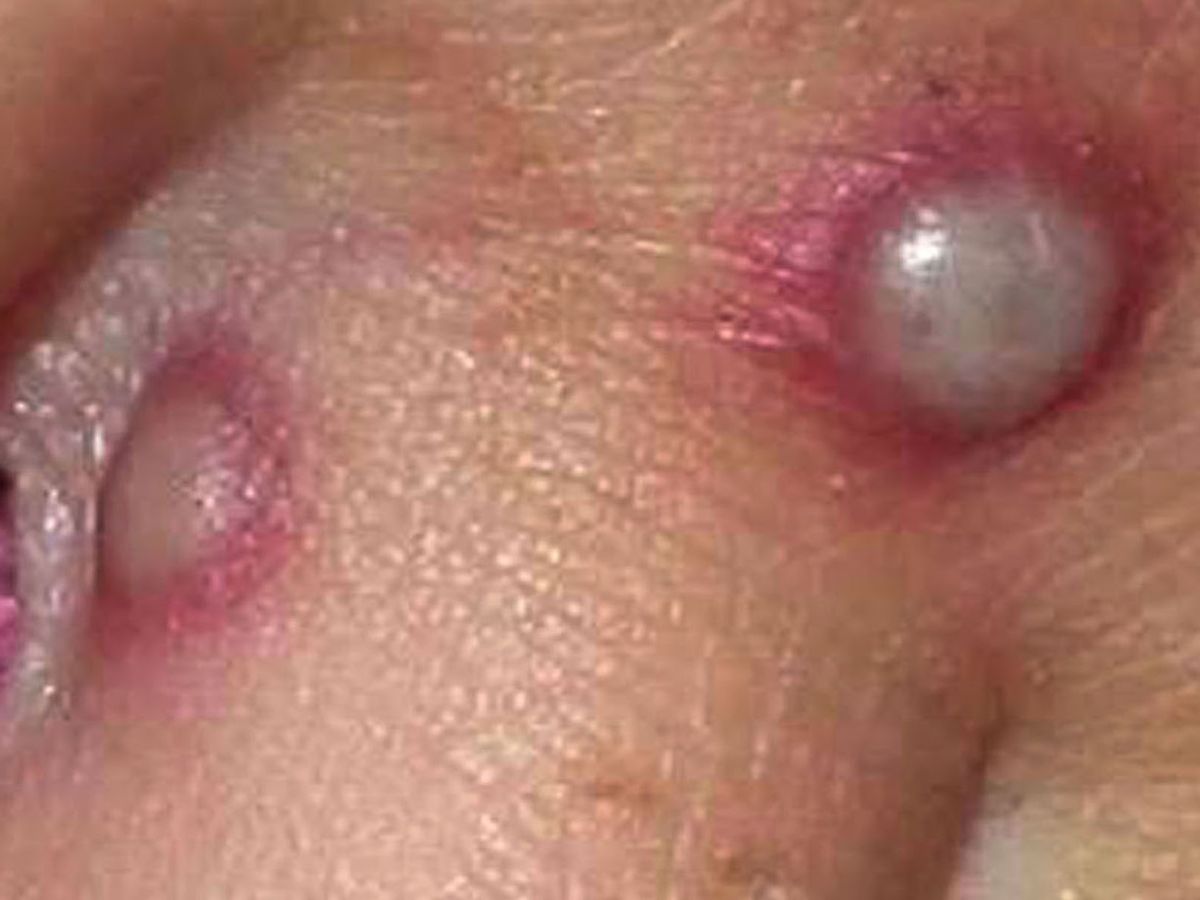
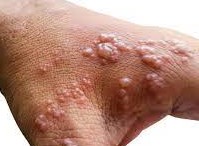
The European Commission has approved a smallpox vaccine to treat monkeypox after the World Health Organization (WHO) declared monkeypox, a global health emergency. Bavarian Nordic said the European Commission has extended the marketing authorization for the smallpox vaccine ‘Imvanex’ to include protection from Monkeypox.
The approval is valid in all the member nations of the European Union, as well as in Norway, Liechtenstein, and Iceland. The WHO has declared the monkeypox outbreak, which has affected around 16,000 people in 72 countries, to be a global health emergency.
Imnavex has been approved in the European Union since 2013 for preventing smallpox, and it was also considered a potential vaccine for monkeypox because of the similarity between the monkeypox virus and the smallpox virus.
According to the data, monkeypox is less dangerous and contagious than smallpox, which was identified in 1980. The European Medicines Agency (EMA) carries out a scientific assessment of drugs and gives a recommendation on whether a medicine should possibly be marketed or not.
Although, under European Union law, the EMA has no power to actually allow and permit marketing in different European Union countries, and it is the European Commission, which is the authorizing body, and takes a decision based on the recommendation of the EMA.